Osmosis is a natural process for which diluted solutions or lighter solutions spontaneously pass in more concentrated solutions through semi-porous diaphragms. The phenomenon is the same for human beings and for all living creatures: the semi-porous diaphragms are made up of cellular fibres. Plants, for example, absorb water and nourishment from the ground, thanks to osmosis.
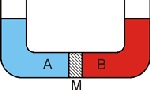
Water tends to pass through the diaphragm, in that it passes from the fluid of a more diluted solution to a more concentrated solution.
For example, soft water will always tend to permeate through an osmotic diaphragm to mix with a more concentrated solution of salty water or sea water.
As soon as the water passes through the diaphragm, the pressure decreases on the less concentrated side. Simultaneously, the pressure of the more concentrated solution increases until a balance is reached, which stops the flow through the diaphragm.
The difference in pressure between the two solutions in such a balanced state is called “osmotic pressure”.
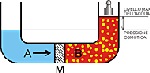
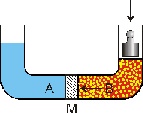
Reverse osmosis is a scientific reversion procedure of the natural osmotic process: all that is needed is the application of a greater pressure than the osmotic pressure to reverse the flow in the semi-permeable diaphragm and therefore to separate the salts and the dissolved solids.
Before this process was possible, a synthetic diaphragm had to be designed, which needed to be highly permeable to water but which also needed to act as an effective barrier against salts and all the other dissolved minerals.
The best solution to your problem
Contact us for a personalized offer
 IT
IT