THEORY OF HARDNESS
Water is never found naturally pure, differing in many respects according to its locality. It is generally distinguished as Soft water and Hard water. For most ordinary purposes soft waters are preferred to the hard, and may be distinguished by the readiness with which they dissolve soap, notwithstanding they may contain considerable foreign matters. Hard waters, on the contrary, holding in solution salts of calcium or other earths, dissolve soap, but subsequently form insoluble compounds with the latter; these waters are unfit for internal use or household or pharmacology purposes. In this respect water is spoken of as having temporary and permanent hardness. Permanent hardness refers to those salts alone that remain in solution after boiling the water. The degree of hardness is defined as the number of grains of calcium carbonate in a specified volume of water.
By more modern usage, 10 mg of calcium carbonate (CaCO3), in 1 litre of water, is called 1 degree of French hardness.
HOW THE SOFTENING PROCESS WORKS
The most effective and safe way of conditioning hard water is the ion exchanging method. For the sake of simplicity, these notes consider the hardness to be just calcium carbonate (CaCO3). Salts dissolved in water dissociate in ions, in other words the molecule is split-up into two parts:
Calcium carbonate (scale) is split-up into calcium ion and carbonate ion:
CaCO3 à Ca++ + CO3 =
Sodium chloride (kitchen salt) is split-up into sodium ion and chlorine ion.
NaCl à Na+ + Cl-
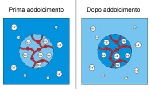
The exchanging resins within the softener fix the calcium ions, freeing sodium ions in return.
When producing softened water, the resin that is loaded with Na+ ions, captures the Ca++ ions from the water and gives back just as many Na+ ions to the water:
hard water enters with scaling CaCO3
soft water is outlet with non-scaling NaCO3
When all the sodium has been let-off, the effect of the resins ends, which are therefore to be regenerated by removing the fixed calcium and loading them again with sodium.
This is obtained by washing with a solution of sodium chlorine in water (brine). When washing with brine, the exhausted resin, loaded with Ca++ ions, captures the Na+ ions from the brine and gives back just as many Ca++ ions to the brine:
- brine enters with NaCl
- water is outlet with CaCl2, that is drained
The best solution to your problem
Contact us for a personalized offer
 IT
IT